06.06.23
Perspectives
Overcoming the limitations of mRNA
The swift development of the first clinical mRNA vaccine awakened the world to the modality’s potential. Following that success, pharmaceutical companies and biotech labs alike are now racing to further improve on the technology and expand its applications.
Despite its success in COVID-19, conventional linear mRNA has some inherent limitations. Linear mRNA provides the cell with instructions for protein production that elicit a burst of protein expression, the results of which are then quickly degraded. Combatting this natural degradation therefore requires a large amount of synthetic material to produce enough protein to induce a therapeutic effect. However, this synthetic material presents as “foreign” to cells, setting off alarm bells that shut down cellular protein production and contribute to the side effects that have limited linear mRNA’s full potential from being realized. While navigating this delicate balancing act was achievable for addressing COVID-19, more complex infectious disease, or chronic illnesses such as cancer and autoimmune disease, remain out of reach for linear mRNA.
Most next-generation RNA technologies seek to bypass this balancing act by limiting expression to certain cell types to reduce the amount of “foreign” material the body sees. However, this approach does not substantively alter the amount or durability of protein production and therefore is ideally suited for incremental improvements upon existing clinical applications.
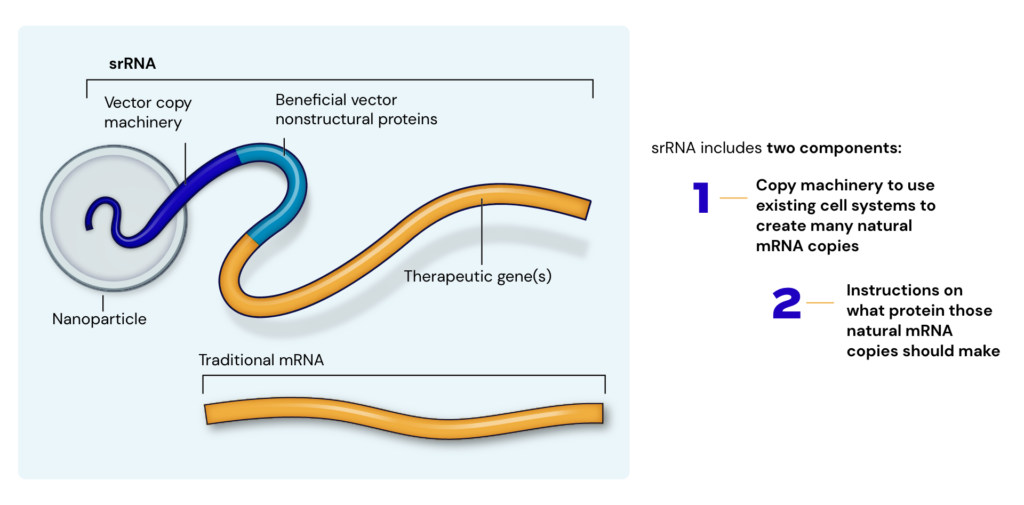
The Power of Replication
In contrast, self-replicating RNAs (srRNA) stand out from other next-generation technologies in their higher capacity for protein production compared to both linear mRNA and circular RNA, increased capacity for genetic cargo, and improved durability of responses. srRNA provide an instruction manual to tell the cell how to produce the natural mRNA encoding the protein of interest. This means only a small amount of “foreign” material is needed to produce large amounts of therapeutic protein, which unlocks applications for RNA treatments in new therapeutic areas and expands applications in existing areas.
This expansion of clinical use cases is enabled by the retention of srRNA bioactivity at lower doses than are possible using linear mRNA or circular RNA. For comparison, animal models have shown that srRNA can be bioactive at one millionth (1/1,000,000) the dose of a linear mRNA. Based on this, we anticipate improved tolerability, as the body is making its own natural mRNA copies from the small amount of administered synthetic srRNA in the therapeutic. Furthermore, it lets us deliver multiple doses over time with less concern of compounding toxicities that hamper the regular dosing of mRNA technology for use beyond vaccination. Lower doses also mean broader accessibility to RNA therapeutics, as manufacturing scales needed to meet global demand will also be lower. Our powerful therapeutics enable us to reach more people than ever before.
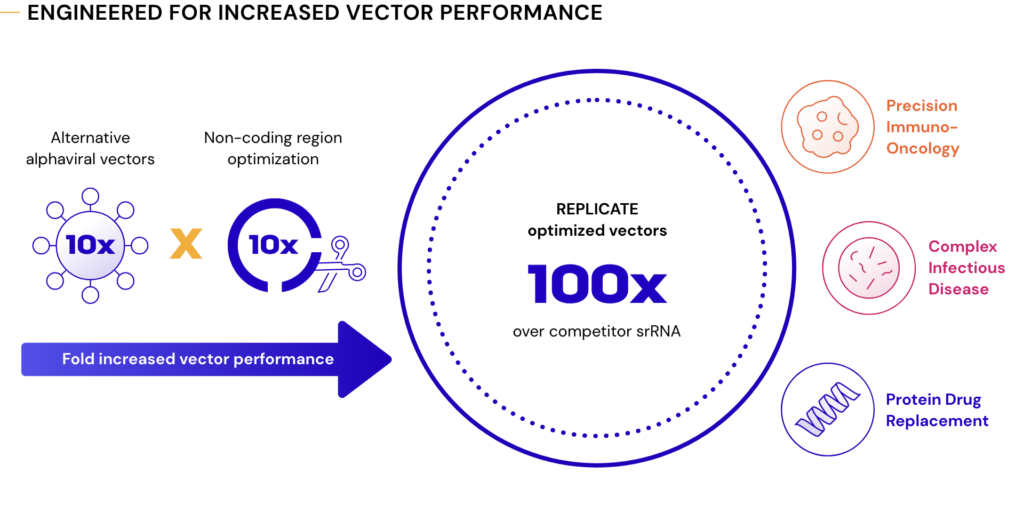
We will test these anticipated advantages clinically this year in infectious disease, the area where RNA technology performance is best understood. Some examples of what we could achieve with improved srRNA technology include:
- Expansion into more complex infectious diseases
- Single dose efficacy could displace technologies beyond RNA
- 50,000,000 vaccine doses from a single 1 liter bioreactor to transform rapid response
- New off-the-shelf precision immunotherapies to create a new paradigm for cancer patients, including for ER+ breast cancer
- Less frequent dosing for proteins of interest that would normally disappear within the body within minutes or hours
- Using the body’s own cells to produce therapeutic endogenous proteins, monoclonal antibodies, engineered proteins, and more
At Replicate, we envision a world where srRNA treatments have reached their full potential as broadly accessible agents that empower the body to fight disease. We are building a platform that expedites the drug development pathway and working on powerful new ways to treat cancer and autoimmune disorders. We are at the cusp of showing this vision can become a clinical reality and look forward to making our approach accessible for all patients.