Our Science
YOUR CELLS’ VERY OWN COPY MACHINERY
Our srRNA Technology
Linear mRNA allows the body’s own cells to produce modest amounts of an introduced protein – an approach that works well for teaching the immune system to respond to infectious disease. Because production is limited, however, this approach requires a large amount of synthetic material to obtain any therapeutic effect. This level of production isn’t sufficient for addressing more complex or chronic illnesses, such as cancer or autoimmune disease.
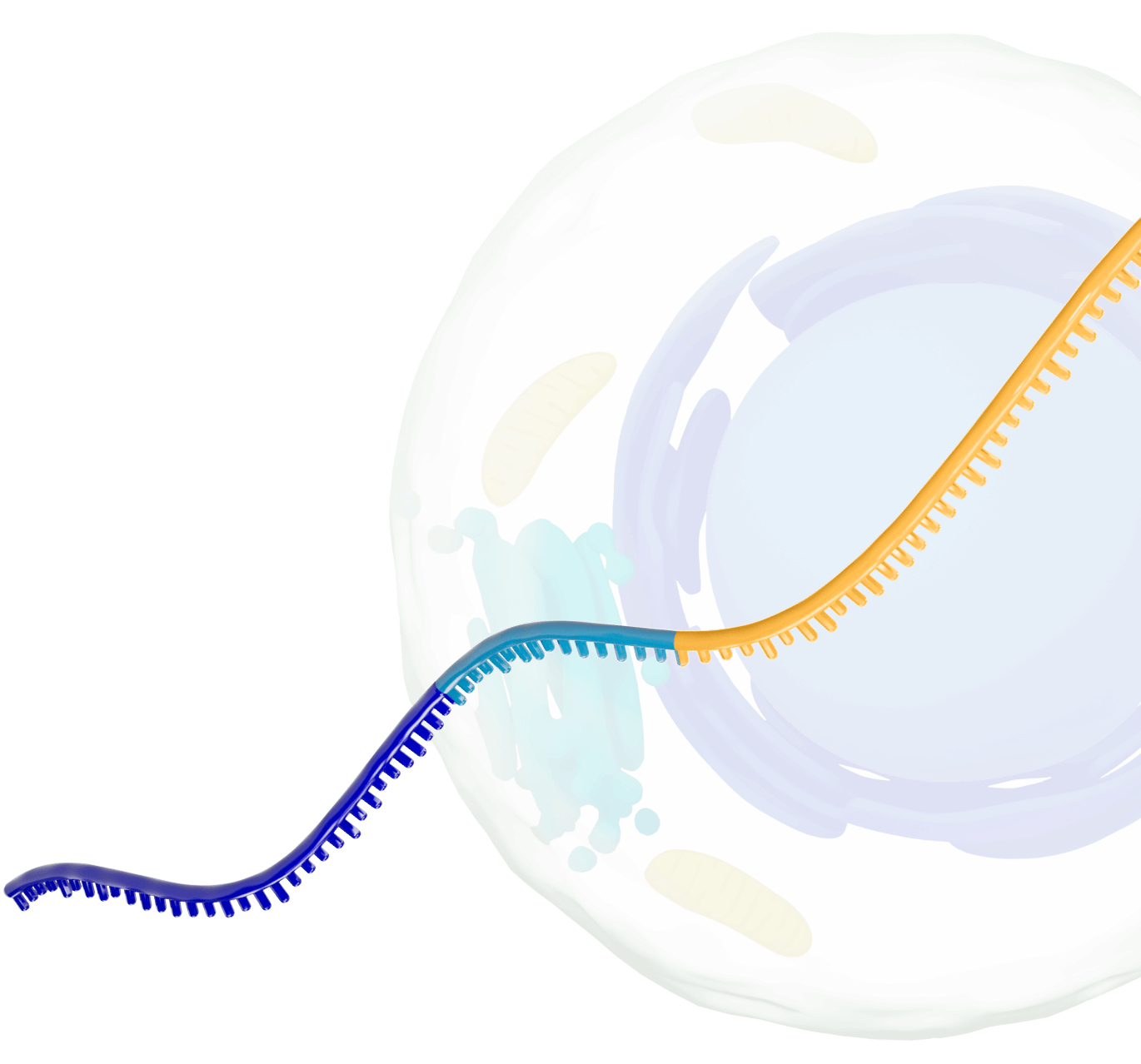
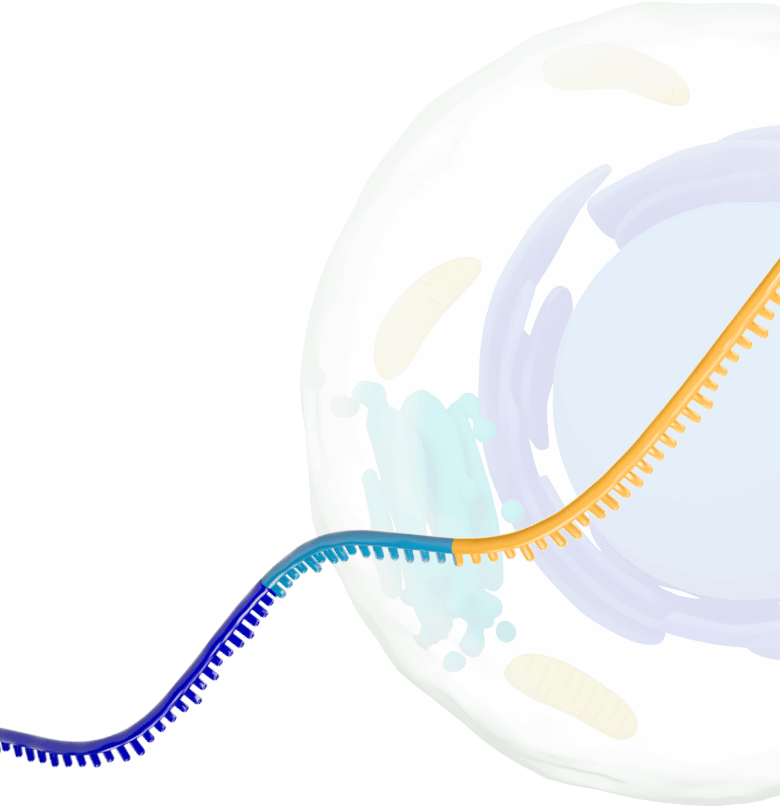
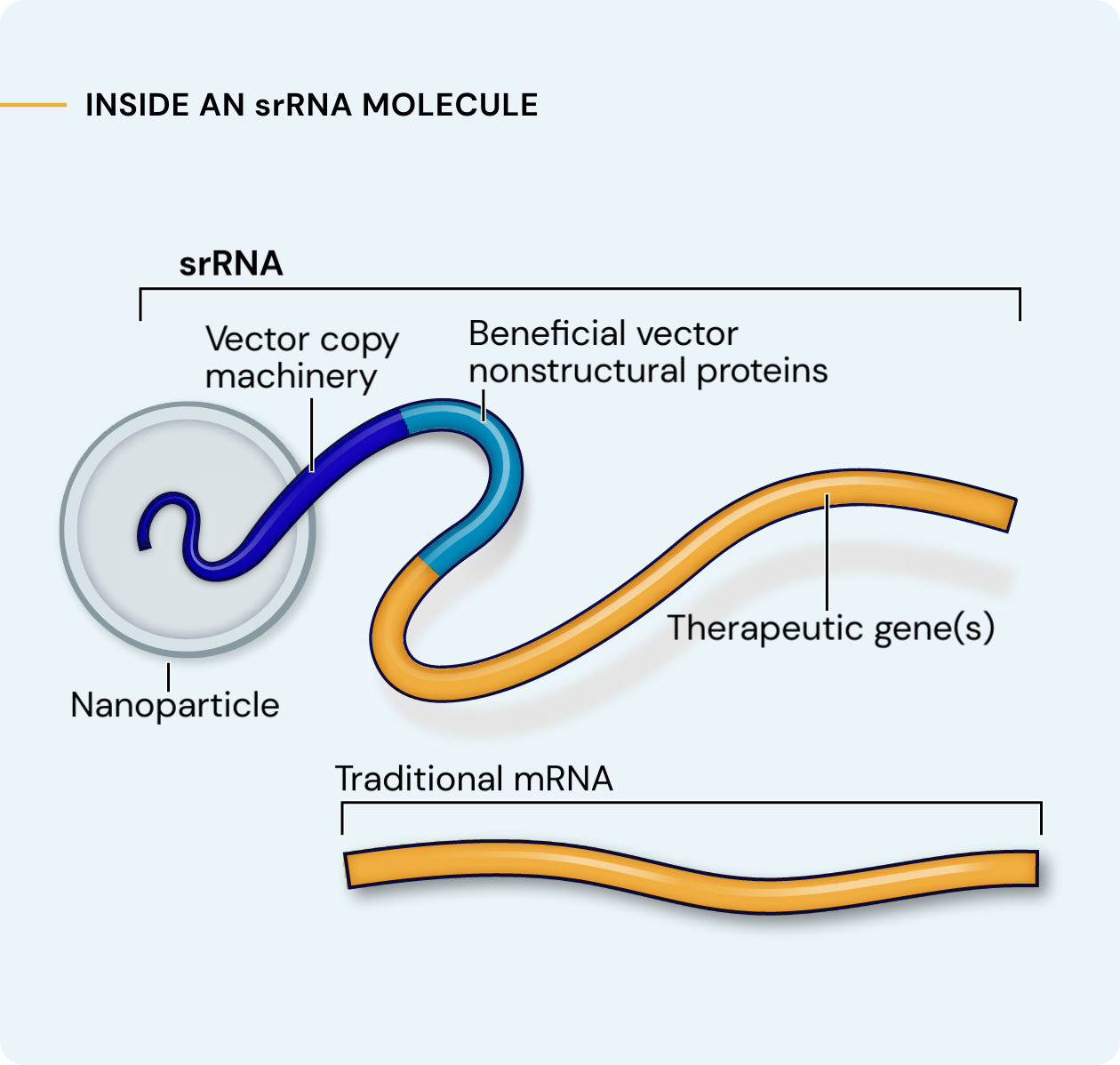
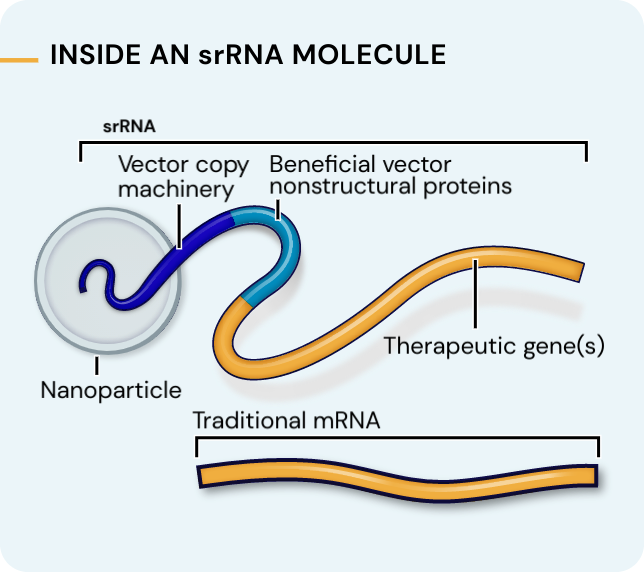
Our platform leverages next-generation self-replicating RNA (srRNA) vectors to amplify protein expression and surpass the bold expectations for the field of RNA treatments. srRNA’s self-limited replication unlocks new opportunities to treat many more patients across a broad range of diseases. In contrast to linear mRNA, we require only minimal amounts of synthetic material to produce enough protein to obtain our therapeutic effects.
srRNA includes two components:
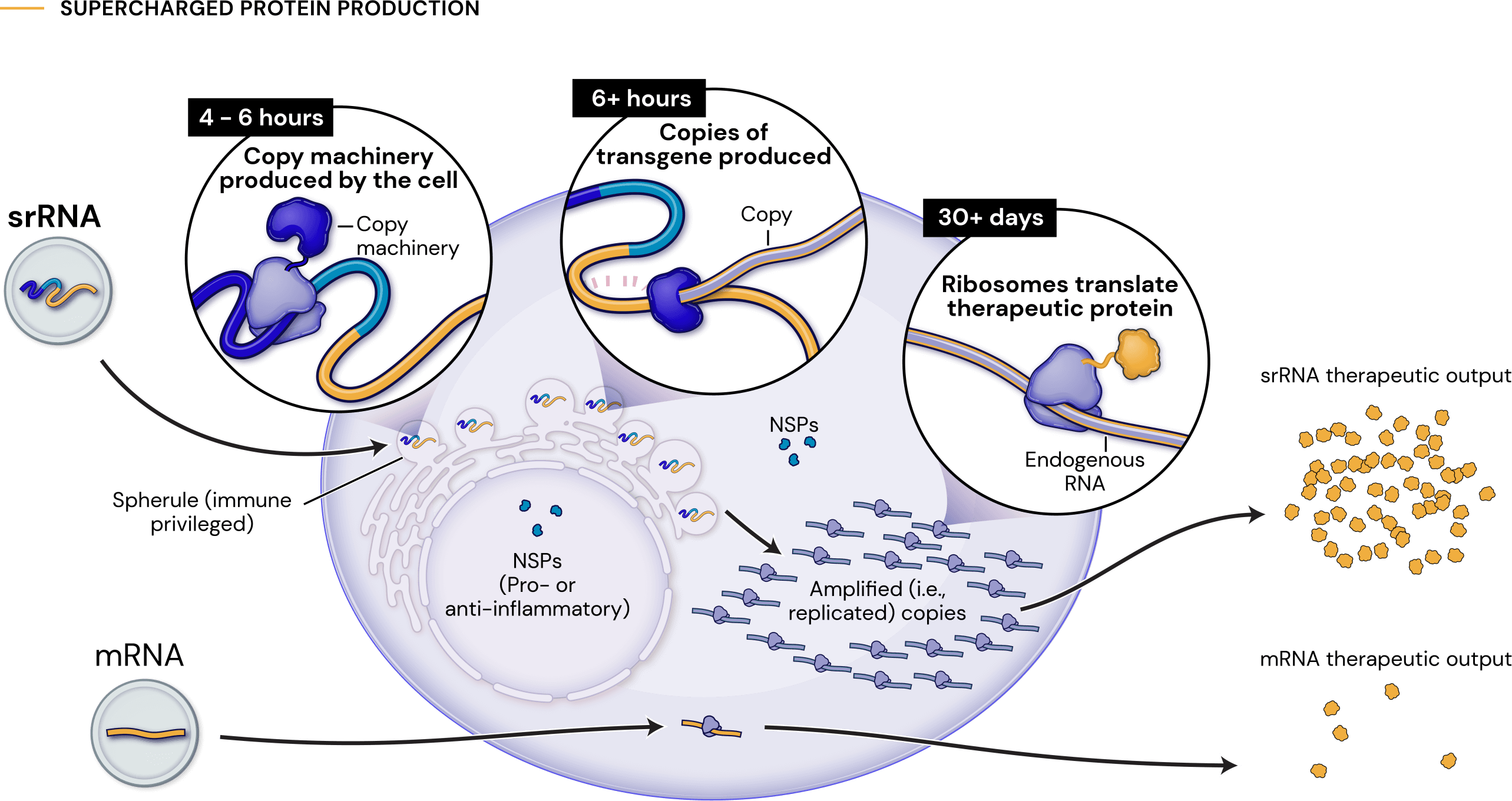
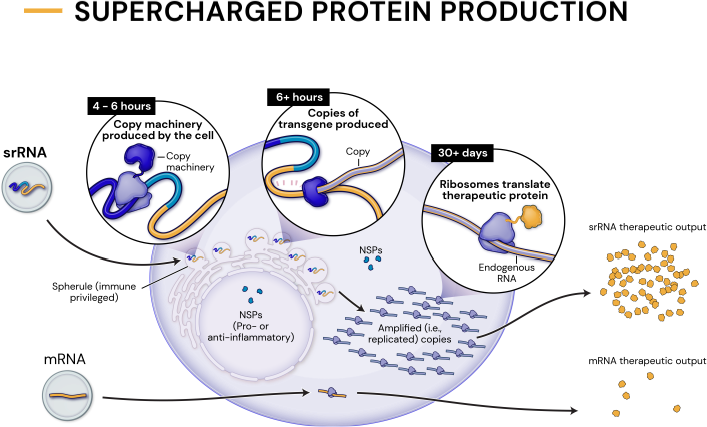

1000x
an approximate 1000-fold expression gain over linear mRNA
100x
a 100-fold gain over standard srRNA
Optimized proteins for srRNA applications, including long or multiple protein transgenes
Selected from a panel of vectors to maximize therapeutic protein production of each unique insert
Chosen from a validated library for either pro- or non- inflammatory indications
Strategic collaborations create best-in-class manufacturing ready for rapid deployment
Broad Applications
With a toolbox of synthetic, engineered vectors derived from new viral species or subtypes, we select the best vector for developing a drug. srRNA can express many types of simple or complex therapeutic molecules, including antigens, antibodies, cytokines, enzymes, and protein agonists or antagonists.
Drug resistance remains the major driving factor behind the clinical failure of targeted therapeutics in oncology. Standard of care (SOC) treatments work by targeting and killing cells that display certain common mutations or structural variations. Faced with targeted destruction, tumor cells develop resistance mutations allowing them to survive and rapidly multiply. Current precision oncology approaches to address acquired resistance mutations have increasingly long and complex drug development timelines and burdensome side-effects.
To address these issues, we have created a novel approach leveraging our srRNA as a targeted immunotherapy, which is called Precision Immuno-Oncology (PIO). PIO targets acquired resistance mutations on cancer cells, which are the major contributors to treatment failure and tumor progression.
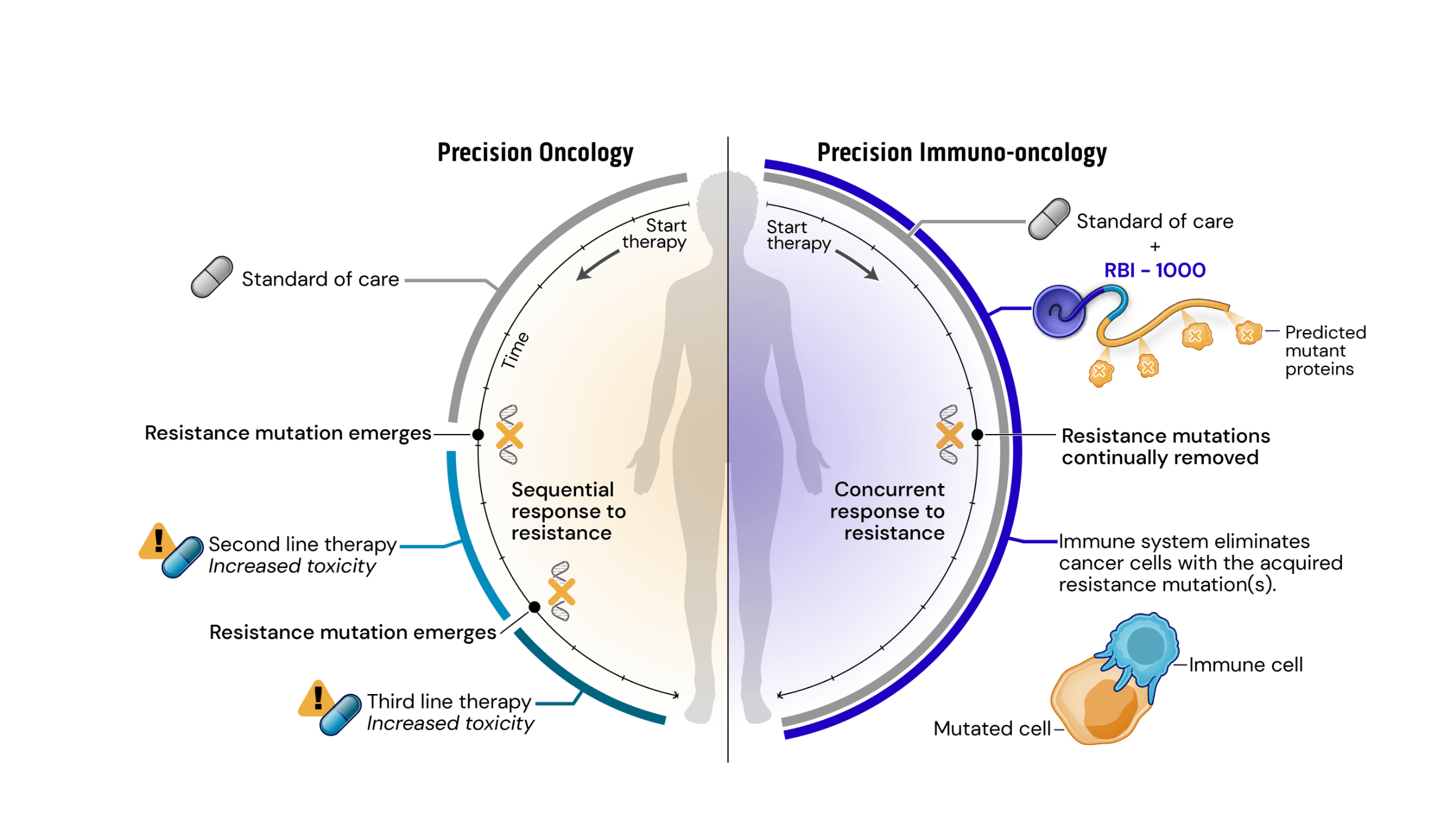
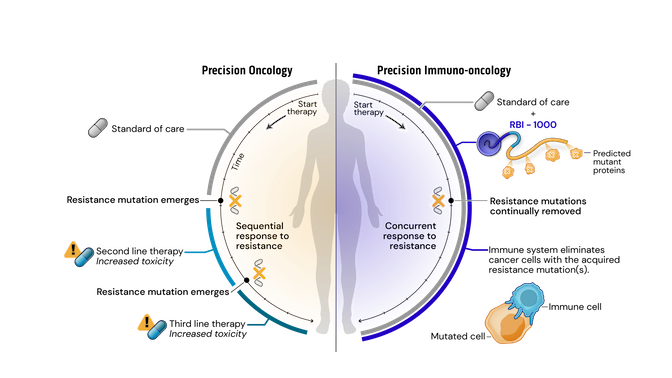
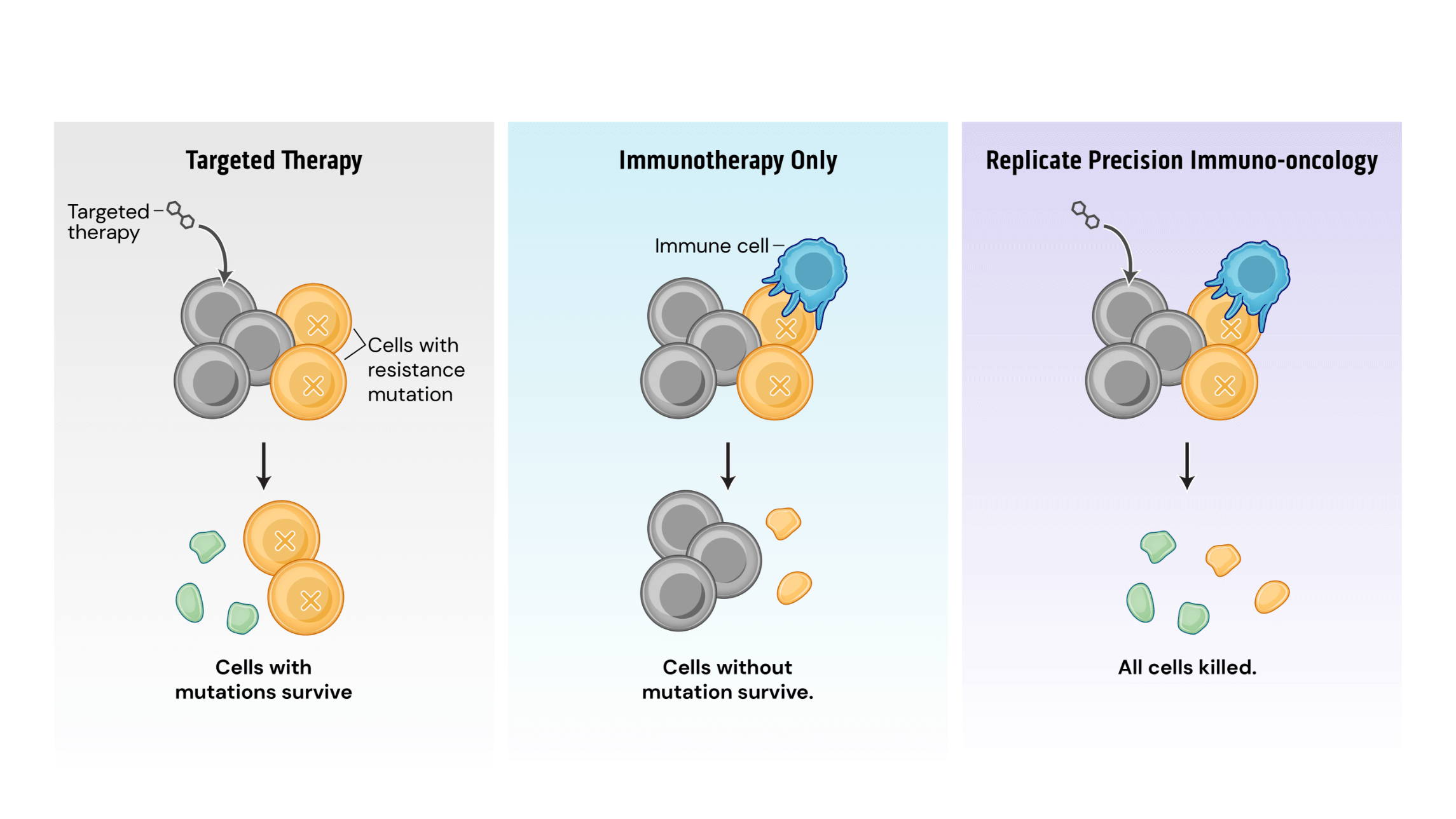
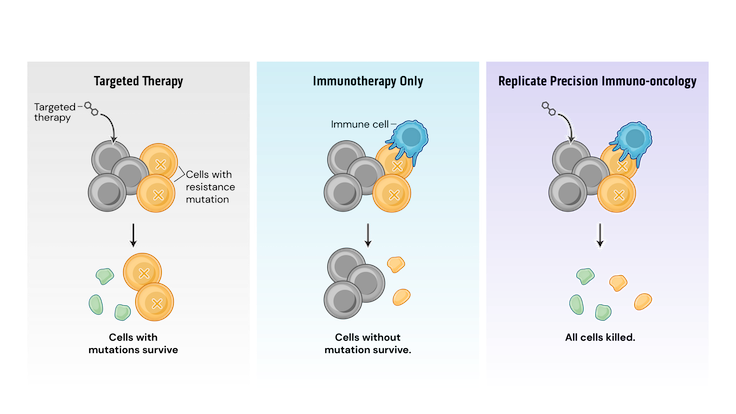
Mechanistically, PIO works by a process termed synthetic immune lethality. When we combine SOC with our precision immuno-oncology srRNAs directed toward predictable resistance mechanisms, we force the tumor into a lose-lose scenario: remain the same and be eliminated by the SOC targeted therapy or mutate and be destroyed by srRNA-trained immune cells.
Our srRNA vectors encode inserts containing multiple potential cancer cell mutations so that a single drug product can address several targets. srRNA vectors generate robust and durable immune cell and antibody responses, successfully inhibiting tumor growth in preclinical models.
Our srRNA technology allows us to rapidly develop vaccine candidates to treat or prevent illness. Extremely low doses that retain bioactivity enable targeting of multiple antigens for a complex infectious disease, or multiple strains for highly multivalent vaccines. These doses also limit side effects to maximize uptake, reduce cost of goods, and accelerate deployment.
Importantly, our potent vectors are designed to maximize the quantity and quality of antibodies and T cells while driving improved durability of protective immunity compared to linear mRNA. Additionally, because of the replication ability of srRNA, a single low dose of a vaccine can provide durable immune protection.
Diseases like autoimmune disorders, inflammatory diseases, or cardiovascular disease can be managed with antibodies or other protein-based drugs. However, synthesis and manufacture of the right protein compounds can be challenging, particularly for large or complex molecules with poor half-lives.
Replicate’s srRNA vectors can deliver instructions for cells to produce their own therapeutic proteins, giving the body the power to manufacture the necessary antibody or protein itself. Durable expression of proteins by our vectors naturally extends the half-lives of encoded molecules, which reduces dosing frequency. By replacing protein drugs with srRNA, we can treat more patients with both rare and common diseases.